
What is a PH meter and its functional features?
A pH meter is a water quality sensor used to measure the acidity or alkalinity of a solution, also called a pH sensor.

A pH meter is a water quality sensor used to measure the acidity or alkalinity of a solution, also called a pH sensor.
A pH meter is a water quality sensor used to measure the acidity or alkalinity of a solution, also called a pH sensor. PH is a measure of the acidity or alkalinity of a solution. It measures on a scale of 0 to 14.

The quantitative information provided by the pH value expresses the degree of the activity of an acid or base in terms of hydrogen ion activity. The pH value of a substance is directly related to the ratio of the hydrogen ion [H+] and the hydroxyl ion [OH-] concentrations. If the H+ concentration is greater than OH-, the material is acidic; i.e., the pH value is less than 7. If the OH- concentration is greater than H+, the material is basic, with a pH value greater than 7. If equal amounts of H+ and OH- ions are present, the material is neutral, with a pH of 7. Acids and bases have free hydrogen and hydroxyl ions, respectively. The relationship between hydrogen ions and hydroxyl ions in a given solution is constant for a given set of conditions, either one can be determined by knowing the other.
More accurate pH measurements are obtained with a pH sensor. A pH measurement system consists of three parts: a pH measuring electrode, a reference electrode, and a high input impedance meter. The pH electrode can be thought of as a battery, with a voltage that varies with the pH of the measured solution. The pH measuring electrode is a hydrogen ion sensitive glass bulb, with a millivolt output that varies with the changes in the relative hydrogen ion concentration inside and outside of the bulb. The reference electrode output does not vary with the activity of the hydrogen ion. The pH electrode has very high internal resistance, making the voltage change with pH difficult to measure. The input impedance of the pH meter and leakage resistances are therefore important factors. The pH meter is basically a high impedance amplifier that accurately measures the minute electrode voltages and displays the results directly in pH units on either an analog or digital display. In some cases, voltages can also be read for special applications or for use with ion-selective or Oxidation-Reduction Potential (ORP) electrodes.

pH electrode technology has not changed much in the past 50 to 60 years. With all the technological advancements of the last 30 to 40 years, pH electrode manufacturing remains an art. The special glass body of the electrode is blown to its configuration by glass blowers. Not a terribly advanced nor “high tech” process but a very critical and important step in the electrode manufacturing. In fact, the thickness of the glass determines its resistance and affects its output.
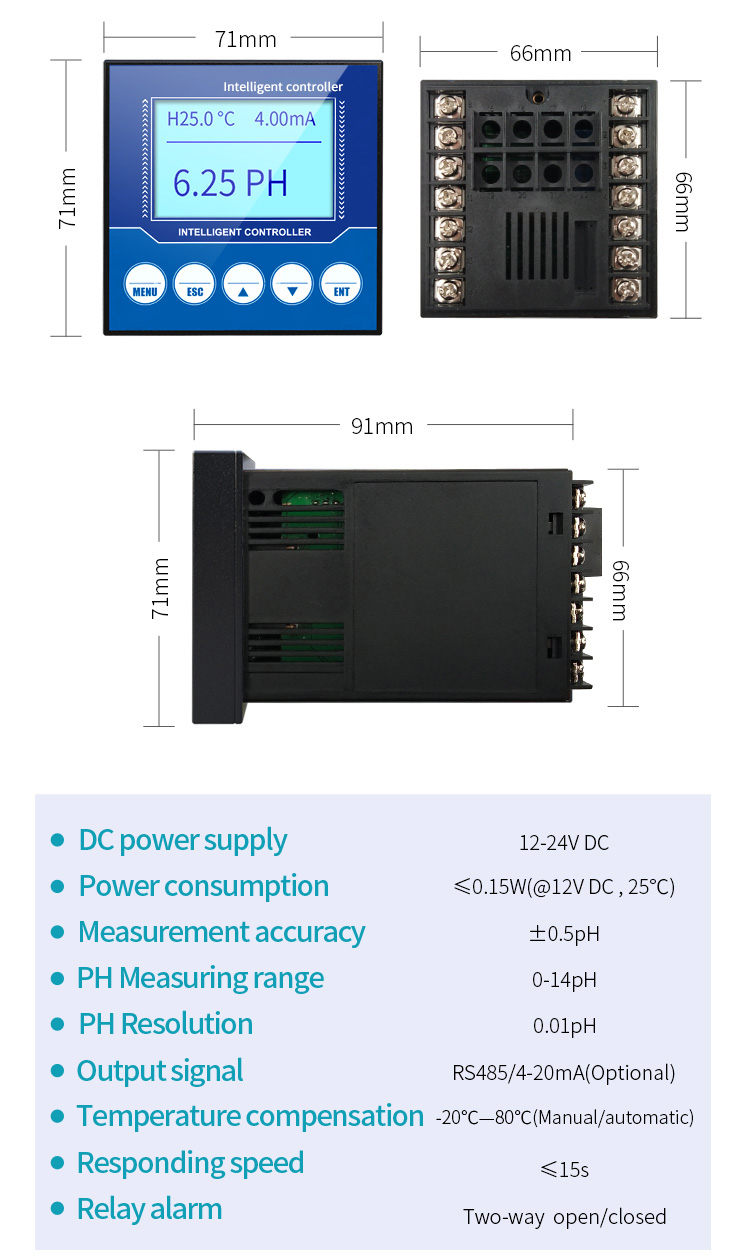
Temperature compensation is contained within the instrument, because pH electrodes and measurements are temperature sensitive. The temperature compensation may be either manual or automatic. With manual compensation, a separate temperature measurement is required, and the pH meter manual compensation control can be set with the approximate temperature value. With automatic temperature compensation (ATC), the signal from a separate temperature probe is fed into the pH sensor, so that it can accurately determine pH value of the sample at that temperature.
Buffers are solutions that have constant pH values and the ability to resist changes in that pH level. They are used to calibrate the pH measurement system (electrode and meter). There can be small differences between the output of one electrode and another, as well as changes in the output of electrodes over time. Therefore, the system must be periodically calibrated. Buffers are available with a wide range of pH values, and they come in both premixed liquid form or as convenient dry powder capsules. Most pH meters require calibration at several specific pH values. One calibration is usually performed near the isopotential point (the signal produced by an electrode at pH 7 is 0 mV at 25°C), and a second is typically performed at either pH 4 or pH 10. It is best to select a buffer as close as possible to the actual pH value of the sample to be measured.
A pH meter is consisted of three different parts: an internal electrode, a reference electrode, and a high input impedance meter. Glass probe often contains the two electrodes — internal electrode and reference electrode. The internal electrode is a Silver wire covered with Silver Chloride (Ag/AgCl wire), and reference electrode is often made up of the same materials. Inside the probe is a buffer solution at pH of 7. Measured pH is the difference in [H+] between the reference buffer inside the probe and the sample solution.
The pH measurements are made by comparing the pH reading of a sample solution to that of a reference solution with defined pH, such as buffers. Therefore, it is important to calibrate the instrument with appropriate buffer solutions before making any measurements. The figure on the right is a simple depiction of a glass electrode used with pH meters.
There are three main types of pH meter available, each with their own applications:
pH electrodes, also known as pen testers, are the cheapest and easiest to use on the market. The electrode is housed in a glass pen-like container with a tip that is sensitive to hydrogen ions. The small size of the electrode meter means that it can be transported and used anywhere, although it does have limitations compared to heavier grade meters. pH electrodes are often used on the go for testing in construction, hydroponics, food manufacturing and pool maintenance.
Handheld pH meters are more heavy duty than their pen tester counterparts. These meters consist of a handheld device for display and calibration features as well as an interchangeable electrode probe component. Handheld pH meters are often used in field work by environmental officers and in aquaculture, agriculture and water treatment industries. Many recent models also feature Bluetooth and wireless technology for easy transmission of data to mobile devices.
Benchtop pH meters are often found in laboratories and professional industries. These are the largest types of meter available and are usually either desk or wall mounted. Benchtop meters provide the highest degree of accuracy when measuring pH levels and are commonly found in food processing facilities, quality assurance, water testing and environmental testing.